Stefan Jungen
MSc thesis 2014, H.P. Lüthi and A. Togni group
"Theoretical investigation of electrophilic trifluoromethylation reactions using hypervalent iodine reagents"
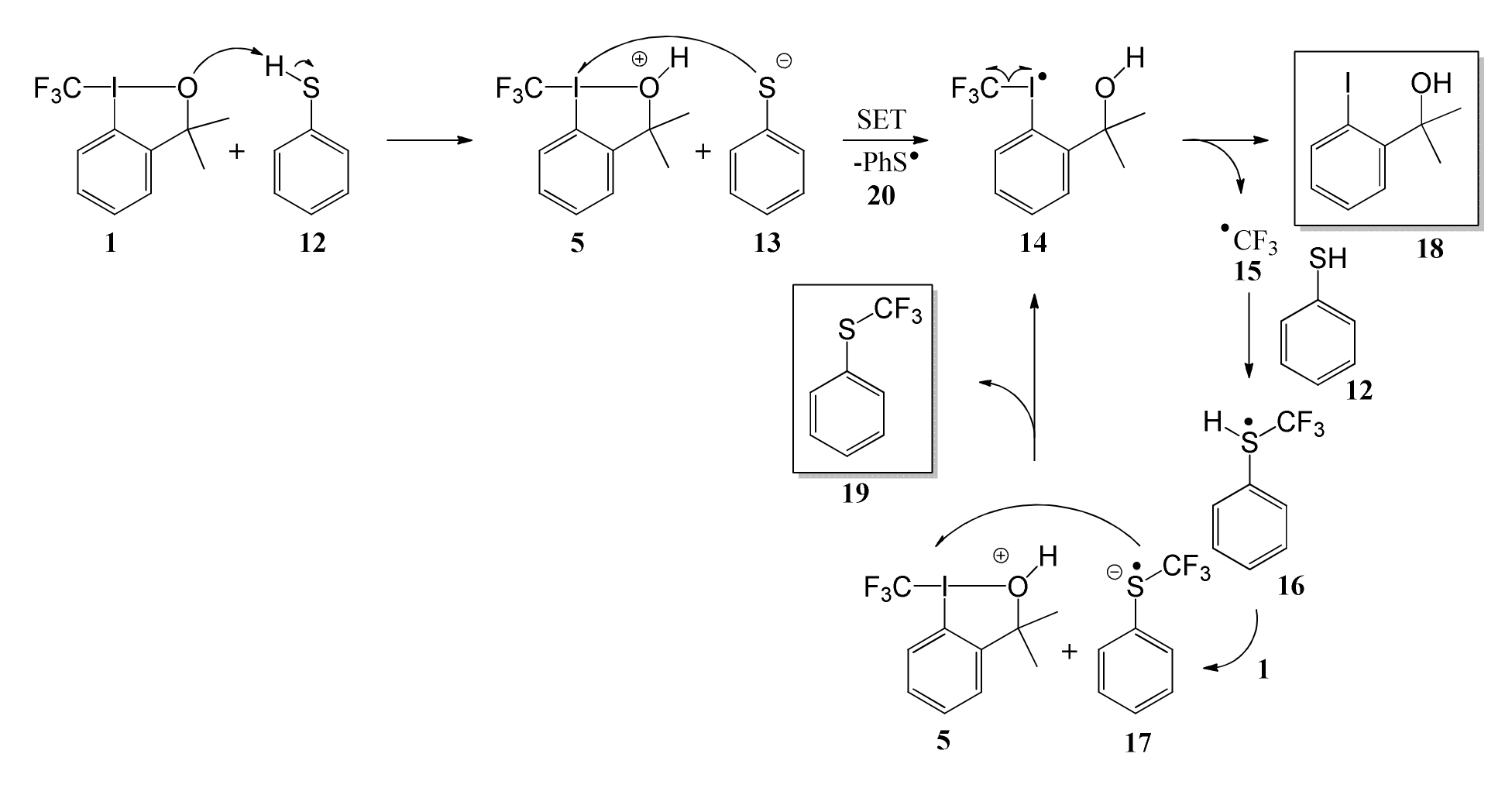
The introduction of hypervalent iodine compounds (iodanes) as electrophilic trifluoromethylation agents by the Togni group has had a remarkable impact on synthetic chemistry. Depending on the nucleophile involved there have been suggested different reaction mechanisms for this process involving reductive elimination, bimolecular nucleophilic substitution or radical procedures. The reaction between 3,3-dimethyl-1-(trifluoromethyl)-1λ3,2-benziodoxole (DMTB, 1) and acetonitrile has been shown before to follow one of the former two mechanisms, most likely reductive elimination. In the first part of this study it is shown by means of density functional theory that the introduction of dispersion correction into calculations considering this reaction does not significantly influence relative energies, structure and mechanistic preference compared to already existing uncorrected computations. Second, a nitrated derivative of DMTB, 3,3-dimethyl-5-nitro-1-(trifluoromethyl)-3H-1λ3, 2-benziodaoxole (DMTB-NO2, 3), is investigated in the context of the same reaction and shown to exhibit higher reactivity than its non-nitrated counterpart DMTB. This is demonstrated to be a consequence of the on average 3 Kcal*mol-1 lower activation energies for DMTB-NO2 systems in gas phase and in the solvent acetonitrile (modelled by IEF-PCM). The main topic and third part of this study is the reaction of DMTB with thiophenol. By investigating this reaction in detail, comparing polar mechanistic possibilities to plausible radical pathways, it could be confirmed computationally to proceed via a radical process as it has been proposed before by the Togni group. In depth analysis of this already existing mechanistic suggestion, however, revealed minor uncertainties and deficiencies of this proposal, which are discussed in this study and attempted to be smoothened by considering different options. A terminal proof for any of these options, however, is not presented in this work, leaving the exact reaction procedure as a subject to debate. In addition, similar investigations were made on the reaction of a related compound -Trifluoromethyl-1,2-benziodoxol-3(1H)-one (OTB, 2) - with thiophenol.